
Emai:marketing@yakkaa.com
業務谘詢專線:400-780-8018
Tel: +1(626)986-9880(U.S. - West Coast)
0044 7790 816 954 (Europe)
Email: marketing@medicilon.com
地址:上海市浦東新區川大路585號
郵編:201299
電話:+86 (21) 5859-1500(總機)
傳真:+86 (21) 5859-6369





© 2023 上海hjc黄金城生物醫藥股份有限公司 保留所有權利 滬ICP備10216606號-3
滬公網安備 31011502018888號 | 網站地圖


業務谘詢
中國:
Email: marketing@yakkaa.com
業務谘詢專線:400-780-8018
(僅限服務谘詢,其他事宜請撥打川沙總部電話)
川沙總部電話: +86 (21) 5859-1500
海外:
+1(626)986-9880(U.S. - West Coast)
0044 7790 816 954 (Europe)
Email:marketing@medicilon.com




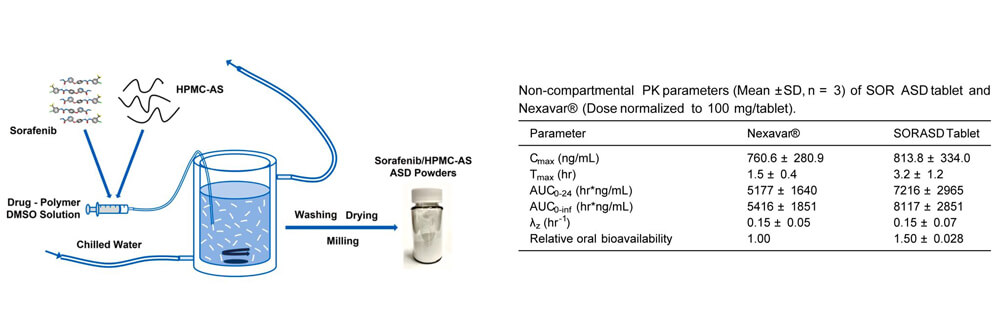
An amorphous solid dispersion (ASD) of Sorafenib (SOR) was used to develop an immediate release tablet with improved oral bioavailability. The SOR ASD tablets exhibited approximately 50% higher relative bioavailability in dogs than the marketed SOR tablet product, Nexavar®. The pharmacokinetic (PK) study of SOR ASD tablets and Nexavar® tablets was performed by Medicilon.
Reference
Sichen Song, et al. Efficient development of sorafenib tablets with improved oral bioavailability enabled by coprecipitated amorphous solid dispersion. Int J Pharm. 2021 Dec 15;610:121216. doi: 10.1016/j.ijpharm.2021.121216.
 相關新聞
相關新聞